Oxygen absorption of copper or brass pigments
Figure 2 shows the results of the determination of copper and brass pigments with 0.5% mass fraction of SMA resin (repeated tests show reproducibility of the gas volume method). Figure 2 shows that the SMA's inhibitory effect on brass pigments is better than that of copper, which is the same as previous results obtained with other types of polymers. Figure 2 demonstrates that as the amount of SMA-2 resin increases, the inhibition of corrosion increases. Figure 3 summarizes the results of adding 0.5% MA and SA resins. MA resin, like SA-1 and SA-2 resins, has an inhibitory effect on the corrosion of copper and brass pigments. SA-3 and SA-4 did not. Therefore, at the pH value of 10, according to the requirements of the suppliers of water-based inks containing metal pigments, like the aluminum-containing pigments, these two SA resins (SA-3, SA-4) cannot suppress the corrosion reaction and cannot be Phenomenon to explain.
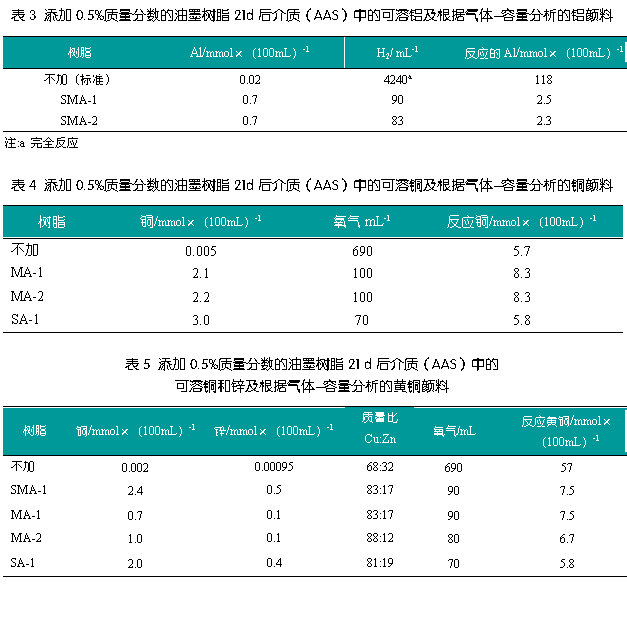
AAS determination of copper and brass pigments
Table 4 shows the results of the AAS determination of the copper pigments, with the copper slightly dissolved in the standard medium. Like aluminum powder, the dissolving power of the copper powder is greatly increased after the ink resin is added. This can also be explained as the formation of a partially dissolved copper powder-resin compound, and the filtered medium appears blue, indicating the formation of a copper compound. By adding ink resin, the proportion of dissolved copper is significantly reduced, and like the above aluminum pigments, the calculation can be performed from the volume of oxygen absorbed.
An approximate linear relationship can be seen if the amount of soluble copper is plotted against the acid value of the ink resin.
The results of AAS determination of brass pigments are listed in Table 5. From the measurement results, three conclusions can be drawn:
(1) In the standard medium, its solubility is very weak. After adding the resin, the amount of dissolved metal quickly increases;
(2) Determined in standard media, the mass ratio of soluble copper and zinc is comparable to that of solid brass pigment (Cu:Zn=70:30). When the resin is added, the dissolution of copper is observed to accumulate. This is contrary to the conclusions of other authors, but it is worth noting that they were tested in acidic media (pH=4) using low-molecular inhibitors. In contrast, polymers (ink resins) are often used as inhibitors in alkaline media today. The results we observed were confirmed by several Indian authors. They also published the use of heterocyclic corrosion inhibitors in alkaline media when the dissolved copper accumulates. Therefore, the compound formed of copper and the resin is assumed to be more soluble in the alkaline medium than the zinc-resin compound.
(3) Similarly, the volume of oxygen absorbed is calculated as follows: After adding the ink resin, the amount of dissolved copper and zinc is lower than that without adding.
Therefore, as a temporary result, it shows that the lower the acid value, the less the metal dissolves. In the past, it was necessary to take low-acid polymers by measuring the gas. However, the inhibition of oxygen and corrosion corrosion of copper and brass is not a sufficient condition.

Figure 6 shows the comparison of Cu and Zn concentrations using 3 similar media. Obviously, compared with copper pigments, brass pigments can dissolve the amount of copper, as well as the soluble copper and zinc in brass. The total amount is low. The solubility of the brass pigment in the medium is lower, which can be explained by the increased stability of the brass.
Absorption of oxygen by organic compounds
In a pure oxygen atmosphere, organic compounds (such as polyethers or resins) may autooxidize, and copper compounds are more likely to catalyze this oxidation reaction. Therefore, a series of oxygen-absorbing tests without metallic pigments were conducted. The medium was deionized with 2.0 mmol/100 mL of ammonium acetate (without wetting agent). In order to simulate the effect of copper compounds, the pH was adjusted to 10 with 2 mmol/100 mL of copper sulfate and TEA, and the oxygen absorption of the stirred media was measured over 21 days. In a further blank laboratory, 2% mass fraction of wetting aid and 0.5% mass fraction of SA-1 resin were added. Blank test results are shown in Figure 7. It is evident that oxygen is significantly absorbed by the medium (TEA and ammonium acetate) (24 ml ie 1 mmol oxygen).
SA-1 resins, particularly wetting aids (polyethers) absorb oxygen. It can therefore be concluded that, as observed in Figures 2 and 3, the corrosion inhibition of copper or brass pigments by ink resins is better due to the absorption of oxygen by different organic compounds in the medium.
in conclusion
In aqueous alkaline ink media, aluminum pigments react to release hydrogen, while inert copper and brass pigments react by absorbing oxygen.
These different types of corrosion reactions can be inhibited by the addition of certain printing ink resins such as styrene-maleic acid resins, maleic acid resins, and some styrene-acrylic polyesters. These ink resins can inhibit the hydrogen evolution of different metals such as aluminum, as well as the oxidation of copper and brass.
As the amount of resin added increases, its corrosion resistance increases.
The above study also showed that MA-1 resin has the lowest acid value and the better its inhibitory effect on metal corrosion. The lower the acid value, the lower the solubility of copper and zinc in the medium, but this is not a sufficient condition for suppressing the oxidation of metallic copper and brass.
In the future, it is possible to synthesize "custom" resins with better performance for different aqueous metallic inks.
Source: Coatings Technology Information Network
Copier Toner For Xerox,Compatible Printer Cartridge,Colorful Compatible Toner Cartridge,Custom Xerox Toner Cartridge
jiangmen jinheng office equipment Co. Ltd. , https://www.jm-jinheng.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)