B-secretase BACE1 (b-site amyloid precursor protein-cleaving enzyme 1) is the rate-limiting enzyme of b-amyloid polypeptide, which is the main component of senile plaques produced by cutting amyloid precursor protein APP to produce Alzheimer's disease An important target for drugs to treat Alzheimer's disease. The existing crystal structure shows that the BACE1 substrate binding pocket is huge and flexible, which makes it very difficult to find its non-peptide small molecule inhibitors. How to fully consider the flexibility of the substrate binding pocket is very important for the design and modification to obtain highly effective inhibitors.
Xu Yechun, a member of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences, determined the structure of different complexes of BACE1 under the same inhibitor and different crystal forms, analyzed the crystal structures of BACE1 and its complexes with inhibitors in all PDB libraries, and analyzed BACE1 performs molecular dynamics simulations to simulate its conformational changes under crystalline conditions. The results show that the three loops around the BACE1 active pocket are very flexible, and the flap region (residues 67-77) is the most flexible. The conformational change that affects Flap can be the specific binding mode of the inhibitor. For example, the hydrogen bond interaction between the inhibitor and the main chain region of Flap can make Flap adopt a closed conformation, thereby covering the active pocket; and when no inhibitor is bound , Flap can adopt the closed conformation but the open conformation in most structures. Different crystalline form accumulations also have an effect on the conformational changes of Flap. For example, molecular dynamics simulations show that in the P6122 crystalline form, crystalline accumulations cause Flap to have more open conformations than closed conformations.
This research on the crystal structure determination, analysis and molecular dynamics simulation system of BACE1 reveals the degree of flexibility of the key area of ​​BACE1 pocket and analyzes the various factors that lead to its flexibility, which provides a very important for the design of further BACE1 inhibitors. Structural information.
Related research results were accepted for publication by Acta Crystallographica Section D (2012, D68, 13-15).
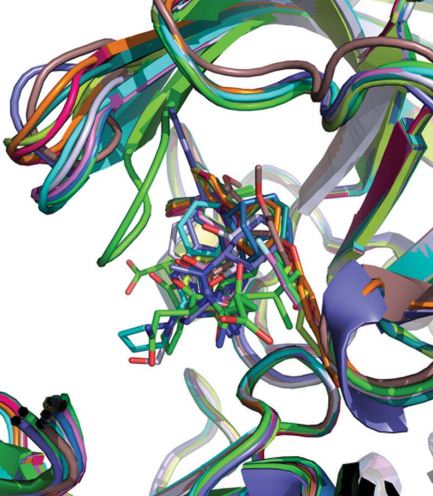
BACE1 key area flap area
Large capacity waterproof backpack, lightweight waterproof computer bag, casual waterproof backpack, PVC waterproof backpack
Dongguan Jinying Handbags Co.,Ltd , https://www.jinyingfashionbag.com
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)