Protein glycosylation is one of the most important post-translational modifications in living systems and plays an important role in life processes such as immune recognition, protein secretion, and signal transduction. Polysaccharides linked to proteins are important carriers for these functions. Especially for monoclonal antibody drugs, the polysaccharide part has an important influence on the biological activity of the drug. Therefore, the development of glycosylation analysis methods with high separation efficiency and good detection sensitivity is of great significance for monoclonal antibody drug analysis.
In response to the difficulties in glycosylation analysis, Waters has developed a hydrophilic interaction chromatography method and a fluorescence-mass spectrometry detection method. ACQUITY UPLC system with fluorescence detector? (FLR) and special polysaccharide analysis (GST) column, has higher resolution than HPLC method. The chromatographic column for polysaccharide analysis is packed with 1.7 μm amide adsorbent, which can effectively separate fluorescently labeled polysaccharides in HILIC mode. UPLC coupled with Fluorescence Detector® analysis of polysaccharides can obtain high resolution and quantitative accuracy, especially for the analysis of positional isomers and co-eluting small peaks; mass spectrometry provides more structure for sugar chain identification information. By comparing with the standard sugar chain retention time, this process can achieve high-throughput qualitative and quantitative polysaccharides to meet the various needs of drug analysis.
1. Chromatographic conditions and separation of labeled polysaccharide samples
The 2-AB labeled polysaccharide mixture can be effectively separated by the HILIC method. For method optimization, the use of a slower narrow gradient can effectively improve the resolution between adjacent polysaccharide peaks in retention time; for other parameters, such as flow rate, buffer concentration, mobile phase pH, and column temperature, etc., generally It also needs to be optimized. Figure 1 illustrates that after using optimized HILIC chromatographic conditions, the complex mixture of 2-AB labeled IgG polysaccharides is well separated, including E1 / E2 and F1 / F2. The gradient elution time used in the experiment was 45 minutes, including the steps of column cleaning and re-equilibration. In general, the total analysis time of a sample is within 1 hour. Therefore, compared with the HPLC method using 3.0-μm filler, the UPLC chromatography method using 1.7-μm filler not only has better separation effect, but also has shorter running time. A 2.1 x150 mm column was used in the experiment. In Figure 1 (B), mannose 5 (peak C) and mannose 6 (peak H) can be successfully separated from adjacent polysaccharide peaks, which solves the problem of co-elution.

2. Quantitative and structural identification of 2-AB-labeled polysaccharides
Since polysaccharides can achieve baseline separation in HILIC mode, various isomers, such as positional isomerization of terminal sialic acid, can be well separated. Therefore, the integration of the peak area under the fluorescence detector enables quantitative analysis of various sugar chains. From the MS spectrum, high mannose glycoforms in polysaccharide samples account for a high proportion, and both complex and hybrid sugar chains can also be identified. Various sugar chains with neuraminic acid can also be identified, indicating that this method is suitable for the analysis of various polysaccharide complexes. In addition to molecular weight, we can further confirm the structure of polysaccharides by MS / MS spectra.
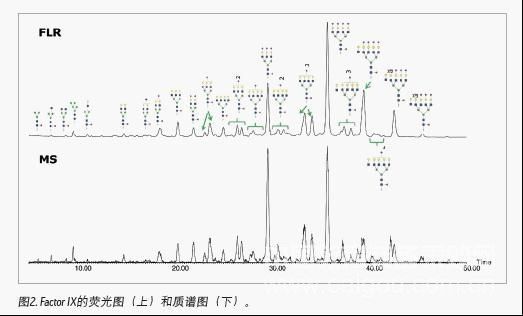
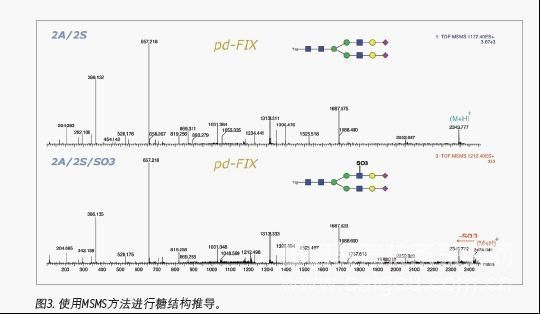
The analysis results of the 2-AB-labeled IgG polysaccharide mixture fully demonstrate that Waters provides a mature glycan analysis scheme, and the quality control of the corresponding chromatographic column uses the 2-AB-labeled IgG polysaccharide mixture. The ACQUITYUPLC system significantly shortens the analysis time, reducing the separation gradient that takes 2 hours or even 3 hours on conventional HPLC to 1 hour. In addition, Waters provides the overall solution of UPLC-FLR-MS to analyze polysaccharides very effectively. In addition to providing molecular weight information, it can also deduce sugar structure, which greatly reduces glycosylation analysis in biopharmaceutical research Difficulty.
experiment process:
1. 2-AB labeled sugar chain
Use GlycoPro le kit, Prozyme
When using the kit to label 2-AB sugar chains, follow the company's instructions except for the following steps.
1. Use 50 μl of labeled reaction solution
2. 65 degrees reaction for 4-5 hours
3. Process the sample according to step 4 to remove excess labeling reagent
Use Sigma reagents
1. Prepare 30% acetic acid in DMSO (30 μl glacial acetic acid, 700ul DMSO)
2. Prepare the 2-AB solution according to the ratio of 20: 1 (v / w) (if you need 20mg 2-AB, use 400μl of 30% acetic acid in DMSO)
3. Mix the 2-AB solution with sodium cyanoborohydride at a ratio of 16.7: 1 (v / w) to prepare a labeled reaction solution
4. Dissolve the obtained sugar chain with 50μl of labeling reaction solution and reflect it at 65 degree shaking for 4-5 hours
5. Treat the reaction solution according to step 4 to remove excess labeling reagent
2. Use MassPrep Hydrophilic Sample Processing Plate to remove excess labeling reagent
Required solution: MiniQ pure water, 90% acetonitrile ACN, 10 mM ammonium acetate Tris, 20% ACN
1. Activate the sample processing plate, add 200μl MiniQ pure water to the sample processing plate, then add 200μl 90% ACN, repeat 90% ACN
2. Pipette 50μl of the labeling solution and add 450μl of ACN (if there is a precipitate, please do not centrifuge, so as not to reduce the sugar chain recovery rate), because the volume of each well on the plate is 200μl, you can divide the sample into four parts
3. Add the sample to the processing board and set the vacuum level to low (pressure 250-500 mmHg) to ensure that the sample has sufficient time to interact with the HILIC matrix; if the solution does not move on the plate, the vacuum can be increased appropriately
4. Wash the treatment board twice with 90% ACN
5. Change to a sample collection plate, elute with 200 μl of 10 mM ammonium acetate Tris, 20% ACN, and transfer the eluate to a 1 ml centrifuge tube
6. The lyophilized sample of sugar chain solution after lyophilization and labeling was reconstituted in 20 μl of 50% ACN, transferred to UPLC sampling bottle after 5 min of ultrasound, and 5 μl was injected.
references
(1) Martin Gilar, Ying-Qing Yu, Joomi Ahn, and Hongwei Xie. Analysis of Glycopeptide Glycoforms in Monoclonal Antibody TrypticDigest using a UPLC HILIC Column
(2) Hongwei Xie, Weibin Chen, Martin Gilar, St John Skiltonand Jeffery R. Mazzeo. Separation and Characterization of N-linkedGlycopeptides on Hemagglutinins In A Recombinant Influenza Vaccine
(3) Joomi Ahn, Ying Qing Yu and Martin Gila.r UPLC Hydrophilic Interaction Chromatography (HILIC) -fluorescence detection method for analysis of 2-AB labeled polysaccharides
Generally, the main parameters are determined according to the requirements of the material handling system, the conditions of the loading and unloading sites, the relevant production process and the characteristics of the materials.
â‘ conveying capacity: Conveyor capacity refers to the amount of material transported per unit of time. In the delivery of bulk material, the hourly delivery of material mass or volume calculation; in the delivery of articles into pieces, the number of hours per hour delivery.
â‘¡ conveying speed: improve the transmission speed can improve the transmission capacity. When the conveyor belt is used as a pulling member and the conveying length is large, the conveying speed is increased. However, high-speed operation of the belt conveyor should pay attention to vibration, noise and start, braking and other issues. For the chain as a tractor conveyor, conveyor speed should not be too large to prevent increased power load. At the same time the process of operation of the conveyor, conveyor speed should be determined in accordance with the production process requirements.
â‘¢ component size: conveyor component size, including conveyor belt width, strip width, hopper volume, pipe diameter and container size. The size of these components directly affect the transport capacity of the conveyor.
â‘£ conveying length and inclination: the length of the transmission line and the size of the angle of a direct impact on the total resistance of the conveyor and the required power.
Conveyor System,Conveyor Belt Systems,Mini Conveyor System,Motorized Conveyor System
Shandong Sinolion Machinery Corp., Ltd. , https://www.sinolion.cc
![<?echo $_SERVER['SERVER_NAME'];?>](/template/twentyseventeen/skin/images/header.jpg)